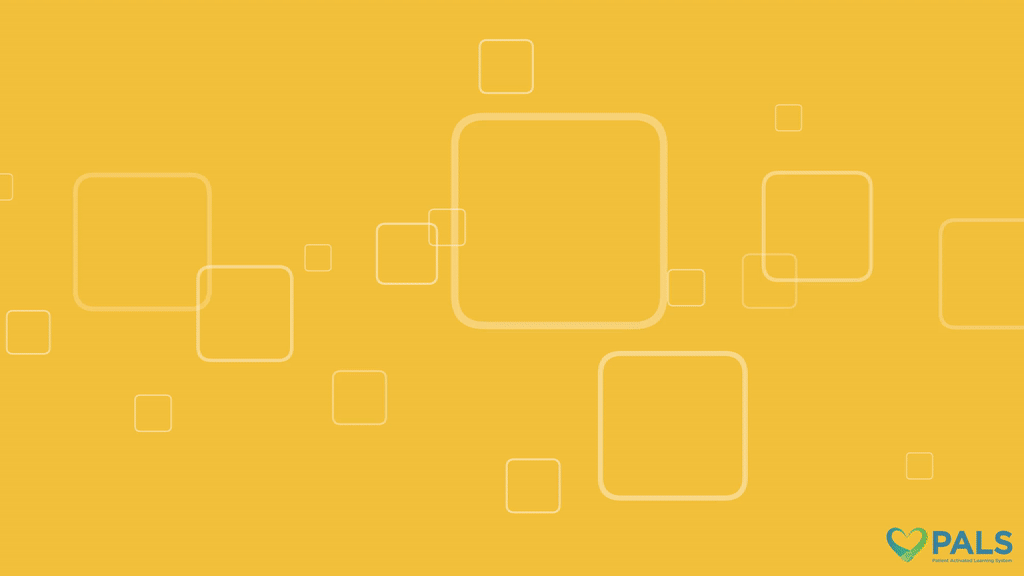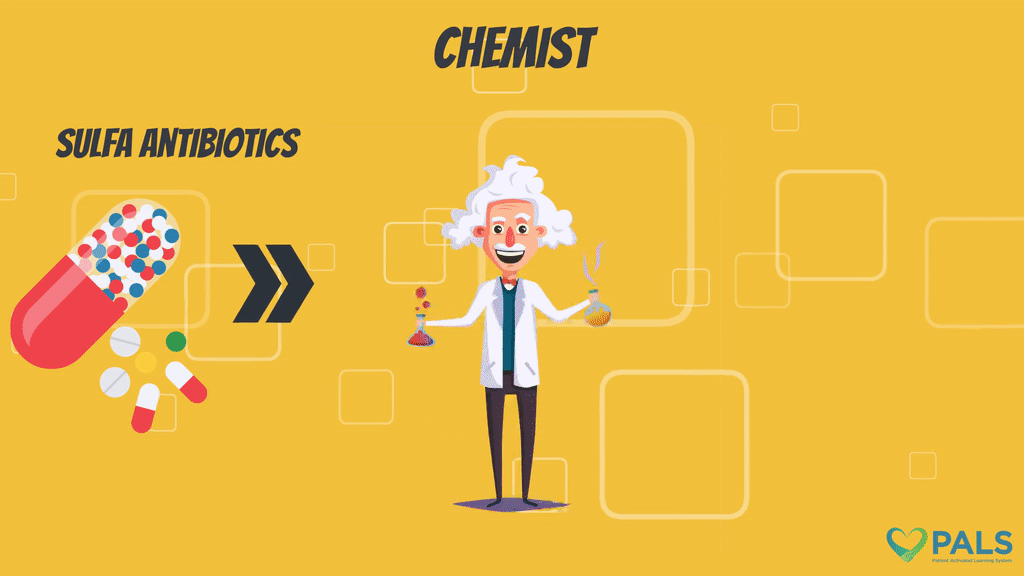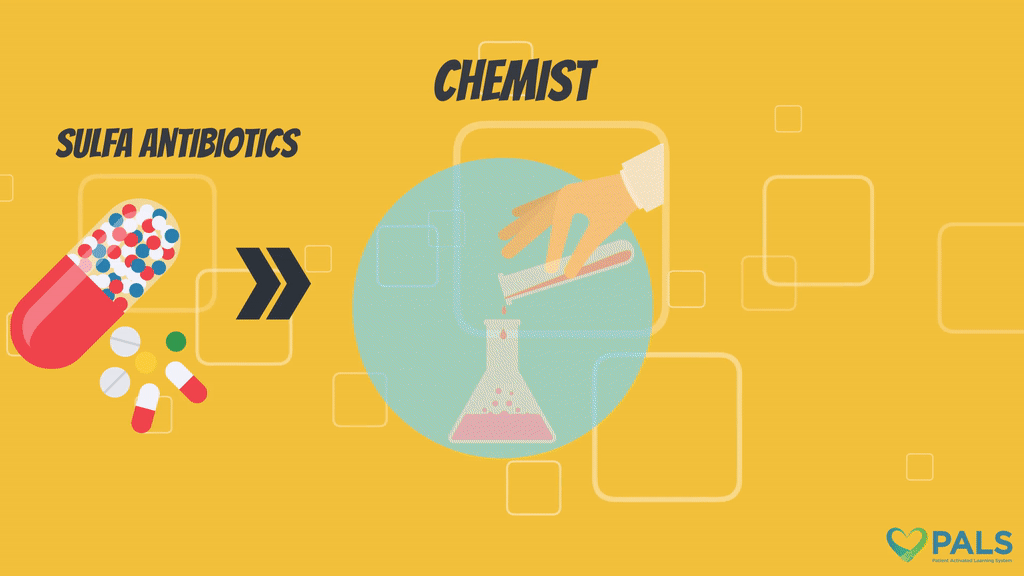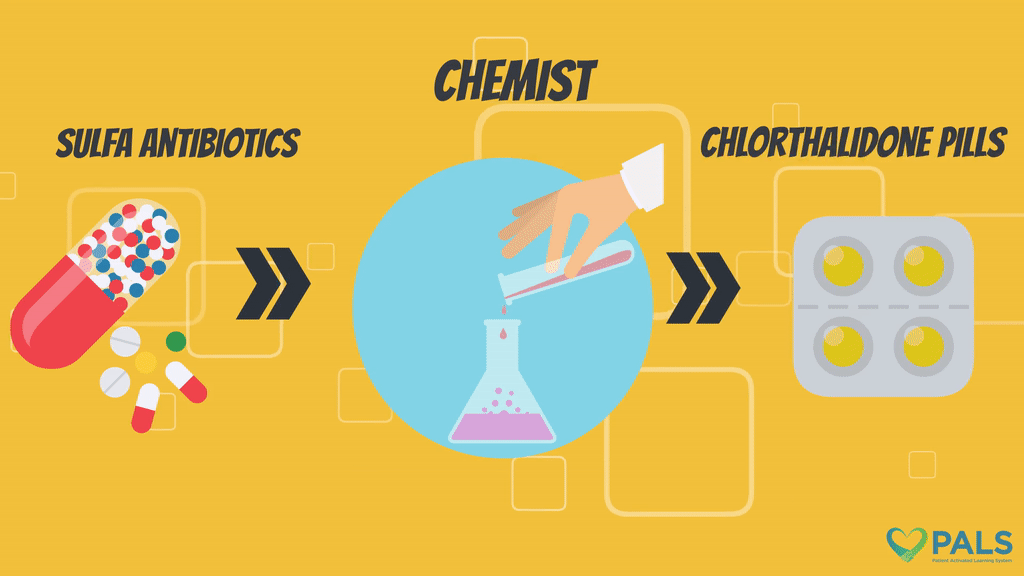
Chlorthalidone, or 2-chloro-5-(1-hydroxy-3-oxo-2H-isoindol-1-yl)benzenesulfonamide, is a synthetically made thiazide-like diuretic.1
Thiazide diuretics originally evolved from sulfanilamide, a derivative of sulfonamides, known to inhibit the enzyme carbonic anhydrase.2 Sulfanilamide was first synthesized by Paul Gelmo in 1908 and used as a dye intermediate.3 Sulfanilamide’s antimicrobial properties were later discovered by Gerhard Domagk.4
In the mid 1940’s, the antihypertensive effects of sulfanilamide was uncovered through the works of Robert Pitts and William Schwartz.5 However, sulfanilamide’s carbonic anhydrase inhibition caused bicarbonate depletion, leading to metabolic acidosis.5 In search of an effective antihypertensive that did not cause an electrolyte disturbance, researchers at Merck developed the first thiazide diuretic, chlorothiazide, in 1958.6 One year later, the chemical structure of chlorthalidone was first described by Graf et al. of the pharmaceutical company Ciba-Geigy.7
There are several methods of synthesizing chlorthalidone.8-11 Chemists may utilize the diazotization process (nitrosating primary aromatic amines to diazonium salts) by diazotizing 3-amino-4-chlorobenzophenone-2-carboxylic acid.8 The diazotized product is reacted with sulfur dioxide followed by thionyl chloride and ammonia to produce chlorthalidone.8 An alternative synthesis method involves heating 4-chloro-2’-carboxybenzophenone-3-sulfonyl chloride with thionyl chloride to produce 3-chloro-3-(3’-chlorosulfonyl-4’-chlorophenyl)phthalide.9 The product is then dissolved in chloroform, reacted with ammonia via an ethanol solute and treated with hydrochloric acid to yield chlorthalidone.9
References
- PubChem Compound Database https://pubchem.ncbi.nlm.nih.gov/compound/2732. Accessed Sept. 14, 2017.
- Greger R, Better OS, Knauf H, Mutschler E, Palmer LG. Diuretics. Berlin ; New York: Springer; 1995.
- Gelmo P. Über Sulfamide der p‐Amidobenzolsulfonsäure. Advanced Synthesis & Catalysis. 1908;77(1):369-382.
- Domagk G. Ein beitrag zur chemotherapie der bakteriellen infektionen. DMW-Deutsche Medizinische Wochenschrift. 1935;61(07):250-253.
- Pizzi RA. Developing diuretics: From mercurials to the thiazides, these "urine promoters" have proved to be some of the most prescribed therapeutics. American Chemical Society. 2003;6(2).
- Novello FC, Sprague JM. BENZOTHIADIAZINE DIOXIDES AS NOVEL DIURETICS. Journal of the American Chemical Society. 1957/04/01 1957;79(8):2028-2029.
- Graf W, Girod E, Schmid E, Stoll WG. Zur Konstitution von Benzophenon-2-carbonsäure-Derivaten. Helvetica Chimica Acta. 1959;42(3):1085-1101.
- Sriram D. YP. Medicinal Chemistry. Second ed: Dorling Kindersley of Pearson Education; 2007.
- Osol A, Hoover JE. Reminton's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co.; 1975:871.
- Budavari S. The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ, USA: Merck and Co., Inc.; 1996:367.
- Kumar A, Singh D, Jadhav A, Pandya DN. An efficient industrial process for 3-hydroxy-3-(3’-sulfamyl-4’-chlorophenyl)phtalimidine. Google Patents; 2005.