Bile acid sequestrants prevent bile acids in the intestine from being reabsorbed into circulation. This forces the body to use cholesterol in the blood to manufacture bile, reducing the amount of cholesterol in the blood. Three bile acid sequestrants have been approved by the FDA for use in the US: cholestyramine, colesevelam, and colestipol. They may be recommended for people with very high LDL-C and low triglycerides, if maximally tolerated statins and ezetimibe have been insufficient to reach goals1 or when statins are not tolerated.2
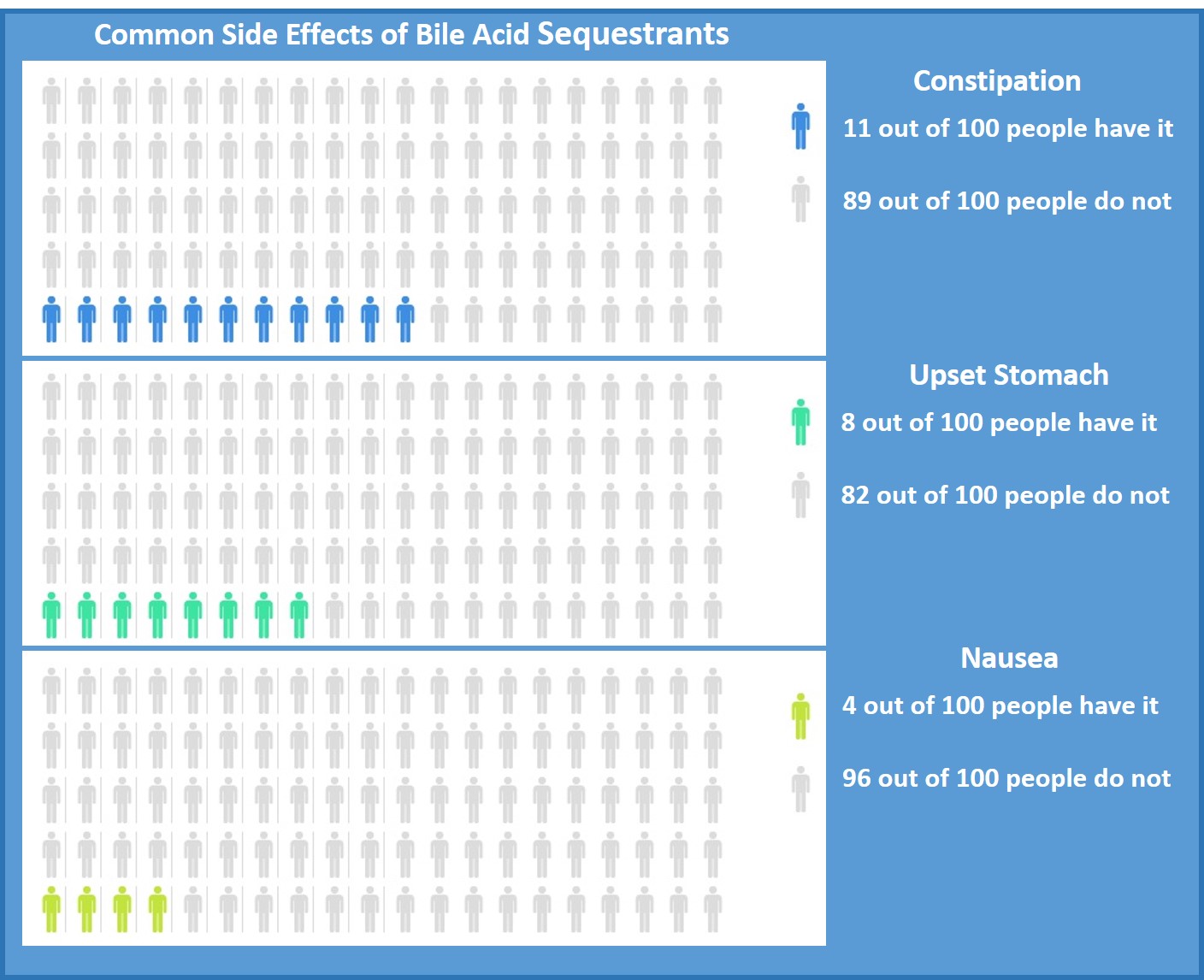
Side effects that have been reported in placebo-controlled studies to occur in ≥2% of people taking colesevelam are included in the table below.3 Though similar side effects are listed for colestipol and cholestyramine their package inserts do not specify their frequency.4,5
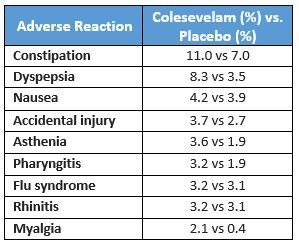
Rare, but serious, gastrointestinal effects have occurred (frequency unspecified) including bowel obstruction, hypertriglyceridemia, pancreatitis, and increased transaminases.3-5
In clinical trials studying the use of colesevelam for lipid-lowering, median triglyceride levels increased by 5% compared to placebo, a difference that was not significant (p=0.42).3 However, seriously high elevations above 500 mg/dL occurred in 4.1% of patients treated with colesevelam compared to 2.0% in patients taking placebo. Significant hypertriglyceridemia can cause pancreatitis and over the long term may increase the risk of cardiovascular disease. For this reason, colesevelam and other bile acid sequestrants are not recommended for use in people with elevated triglycerides.3-5
Compared to other lipid-lowering medication, bile acid sequestrants may be less well tolerated due to side effects associated with the gastrointestinal system. Kamal-Bahl et al. compared rates of medication discontinuation among 233,588 patients taking lipid-lowering medication in a retrospective cohort study using a prescription database over a two-year period.6 The highest rate of discontinuation (68.4% in the first year of use) occurred in patients taking bile-acid sequestrants, compared to 28.3% and 34.4% for statins and ezetimibe, respectively.
References
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol. J Am Coll Cardiol 2018.
- Fischer S, Julius U. Management of patients with statin intolerance. Atheroscler Suppl 2017; 30 : 33-37.
- Colesevelam [package insert]. Parsippany, NJ: Ascend Laboratories, LLC; 2018.
- Colestipol [package insert]. New York, NY: Pfizer Pharmaceuticals; 2014.
- Cholestyramine [package insert]. Pointe-Claire, Quebec: Odan Laboratories; 2016.
- Kamal-bahl SJ, Burke T, Watson D, Wentworth C. Discontinuation of lipid modifying drugs among commercially insured United States patients in recent clinical practice. Am J Cardiol 2007; 99 : 530-534.