All medications cause side effects, but angiotensin receptor blockers (ARBs) are often the best tolerated of the antihypertensive agents.1-3 A meta-analysis of 354 randomized double-blind placebo controlled trials of hypertension treatment with thiazides, beta blockers, angiotensin converting enzyme inhibitors (ACEIs), calcium channel blockers, and ARBs, found that ARBs had no excess symptoms at the standard dose.1 The other medications caused one or more symptoms at the standard dose at rates of 3.9% in the ACEI group (primarily cough), 7.5% in the beta-blocker group, 8.3% in the calcium channel blocker (CCB) group, and 9.9% in the thiazide group. This analysis also found no participants withdrew due to side effects in the ARB group. In comparison, rates of treatment withdrawal due to adverse symptoms (treated minus placebo) were 0.8% (0.3% to 1.4%) for beta blockers, 0.1% for thiazides and ACEI, zero for calcium channel blockers (at half the standard dose), and zero for ARBs at regular doses. The unabridged version of this study gave more detail on symptoms reported for ARBs.4 The prevalence of symptoms severe enough for patients taking an ARB to stop treatment was 0.0% of cases. Out of 26 studies including 7,701 patients on ARBs and 2,803 on placebos, the prevalence of symptoms recognized as being caused by the drugs was:
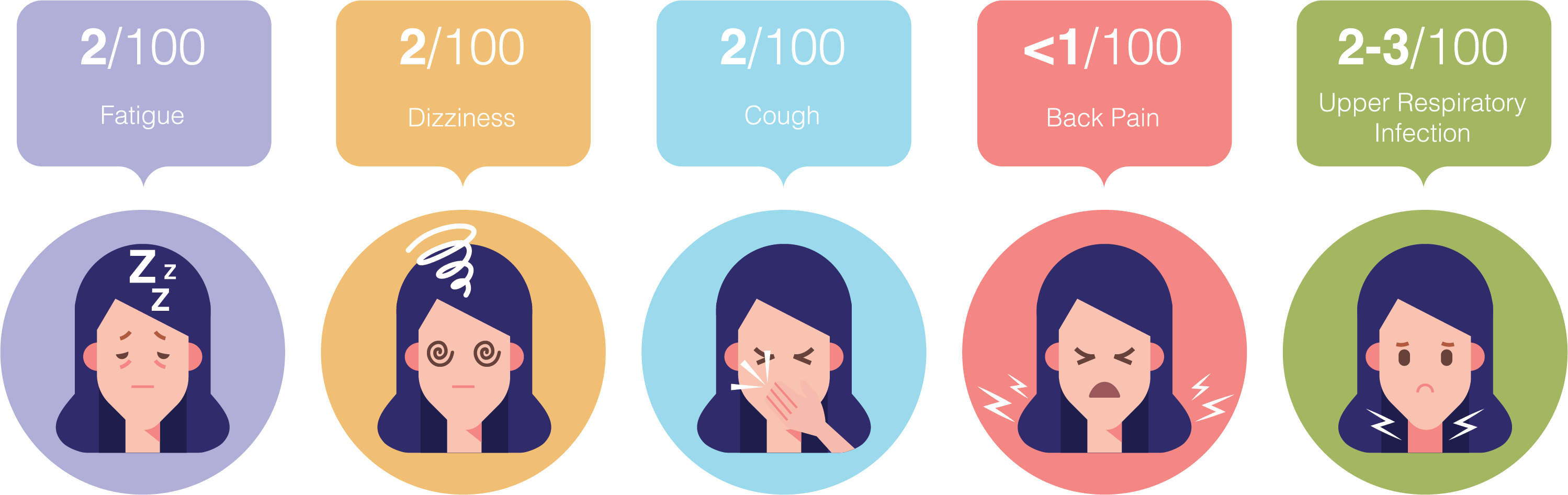
- Back pain (0. 5% ARB vs. 0.7% placebo)
- Cough (1.6% vs. 4.5%)
- Dizziness (2.2% vs. 5.8%)
- Upper Respiratory Tract Infection (2.6% vs. 7.3%)
- Fatigue (1.4% vs. 4.3%)4
ARBs are recommended in several major hypertension management guidelines due to their efficacy and tolerability.3,5-8 They are explicitly suggested as a suitable alternative for people intolerant of ACEI due to side effects, particularly cough.
A 2014 Cochrane meta-analysis found a high level of evidence of a slightly lower incidence of withdrawals from studies due to adverse effects for ARBs as compared with ACEI (risk ratio [RR] 0.83, 95% confidence interval [CI] [0.74 – 0.93]; absolute risk reduction (ARR) 1.8%, number needed to treat for an additional beneficial outcome (NNTB) 55 over 4.1 years).2 This was mainly due to a higher incidence of dry cough with ACEI.
A 1995 double-blind clinical trial of about 2,900 patients compared the safety and tolerability of the ARB losartan potassium against hydrochlorothiazide (HCTZ), atenolol, felodipine ER, and ACEIs for the treatment of systemic hypertension.9 The only symptom reported more frequently with losartan (2.4%) than with placebo (1.3%) was dizziness. Rates of discontinuation due to adverse effects were lower in the losartan (2.3%) and losartan + HCTZ (2.8%) groups than the placebo group (3.7%).
A 2001 randomized, double-blind trial compared the efficacy of four ARBs (olmesartan, losartan, valsartan, and irbesartan) in the control of essential hypertension.10 Of the 588 patients in the treatment phase, 30.6% (n=45) in the olmesartan group reported at least one adverse effect, compared to 32.0% (n=48) of the losartan group, 44.8% (n=65) of the valsartan group, and 35.6% (n=52) of the irbesartan group. Only seven people withdrew from the study due to adverse effects and only two of these were deemed possibly due to the study treatment. The percentage of specific events reported (but not necessarily treatment-related) out of the total number of participants in each group was:
- Upper respiratory tract infection (olmesartan 4%, losartan 4%, valsartan 12%, irbesartan 8%)
- Headache (olmesartan 7%, losartan 6%, valsartan 6%, irbesartan 8%)
- Fatigue (olmesartan 3%, losartan 5%, valsartan 3%, irbesartan 2%)
- Back pain (olmesartan 1%, losartan 5%, valsartan 3%, irbesartan 2%)
- Dizziness (olmesartan 2%, losartan 1%, valsartan 2%, irbesartan 5%)
- Diarrhea (olmesartan 2%, losartan 1%, valsartan 1%, irbesartan 5%)
- Arthralgia (olmesartan 1%, losartan 3%, valsartan 3%, irbesartan 1%)
- Coughing (olmesartan 3%, losartan 1%, valsartan 2%, irbesartan 1%)
- Pharyngitis (olmesartan 0%, losartan 4%, valsartan 1%, irbesartan 1%)
- Influenza-like symptoms (olmesartan 1%, losartan 0%, valsartan 1%, irbesartan 4%)
- Myalgia (olmesartan 0%, losartan 1%, valsartan 4%, irbesartan 0%)
- Toothache (olmesartan 0%, losartan 0%, valsartan 4%, irbesartan 1%)
- Peripheral edema (olmesartan 1%, losartan 0%, valsartan 3%, irbesartan 1%)
- Migraine (olmesartan 0%, losartan 3%, valsartan 0%, irbesartan 0%)10
According to the FDA-approved prescribing information, the most common side effects that can occur in at least 1% of patients receiving an ARB are:11-18
- Dizziness
- Headache
- Fatigue
- Cough
- Cold or flu symptoms
- Muscle, joint, or back pain
- Abdominal pain or diarrhea
References
- Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 2003; 326 (7404): 1427.
- Li EC, Heran BS, Wright JM. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev 2014; (8): Cd009096.
- Powers BJ, Coeytaux RR, Dolor RJ, et al. Updated report on comparative effectiveness of ACE inhibitors, ARBs, and direct renin inhibitors for patients with essential hypertension: much more data, little new information. J Gen Intern Med 2012; 27 (6): 716-729.
- Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess 2003; 7 (31): 1-94.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62 (16): e147-239.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. JAMA 2014; 311 (5): 507-520.
- Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Circulation 2015; 131 (19): e435-470.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017.
- Goldberg AI, Dunlay MC, Sweet CS. Safety and tolerability of losartan potassium, an angiotensin II receptor antagonist, compared with hydrochlorothiazide, atenolol, felodipine ER, and angiotensin-converting enzyme inhibitors for the treatment of systemic hypertension. Am J Cardiol 1995; 75 (12): 793-795.
- Oparil S, Williams D, Chrysant SG, Marbury TC, Neutel J. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension. J Clin Hypertens (Greenwich) 2001; 3 (5): 283-291, 318.
- Micardis [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2014.
- Diovan [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2017.
- Cozaar [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; 2015.
- Edarbi [package insert]. Atlanta, GA: Arbor Pharmaceuticals, LLC; 2016.
- Atacand [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
- Avapro [package insert]. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2016.
- Benicar [package insert]. Parsippany, NJ: Daiichi Sankyo, Inc; 2009.
- Teveten [package insert]. North Chicago, IL: AbbVie Inc.; 2014.

.png)
