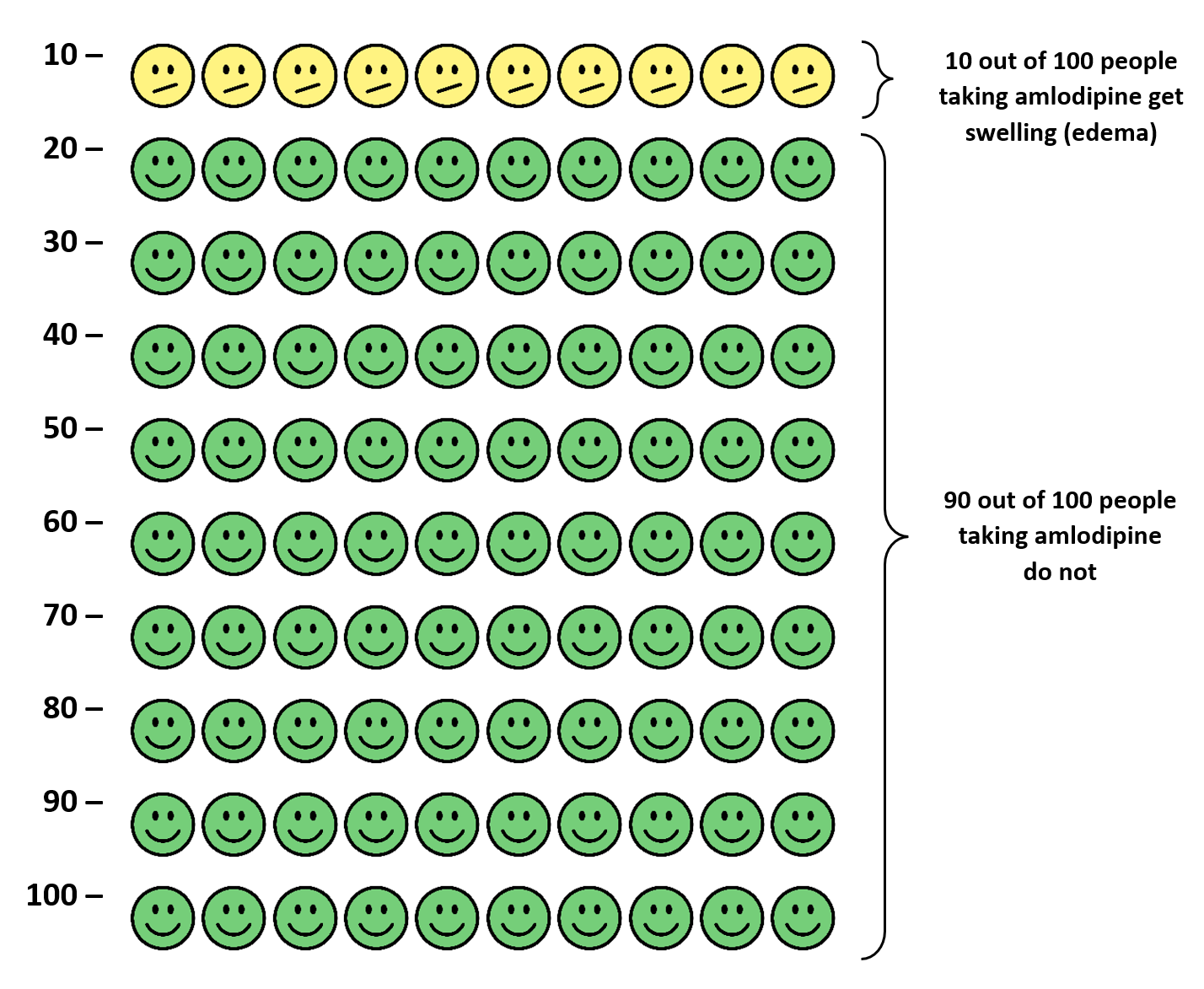
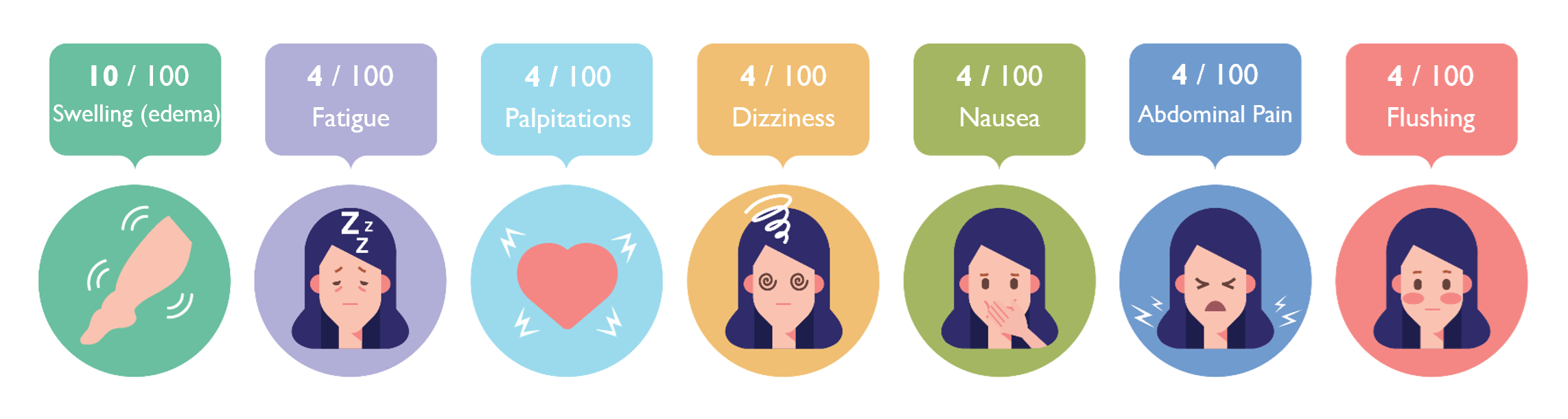
The manufacturer’s drug information reports combined side effect rates in controlled clinical trials comparing amlodipine (N=1,730) to placebo (N=1,250).1 The individual studies included in the analysis are not reported or cited. The incidence of edema, dizziness, flushing and palpitations was dose dependent. At 2.5 mg per day of amlodipine, patients experienced edema, dizziness, flushing, and palpitations at rates of 1.8%, 1.1%, 0.7%, and 0.7%, respectively. At 5.0 mg per day of amlodipine, patients experienced edema, dizziness, flushing, and palpitations at rates of 3.0%, 3.4%, 1.4%, and 1.4%, respectively. At 10.0 mg per day of amlodipine, patients experienced edema, dizziness, flushing, and palpitations at rates of 10.8%, 3.4%, 2.6%, and 4.5%, respectively. In the placebo group, the rate of edema, dizziness, flushing and palpitations were 0.6%, 1.5%, 0.0%, and 0.6%, respectively.
Other side effects reported were headache (7.7% on amlodipine, 7.8% on placebo) fatigue (4.5% on amlodipine, 2.8% on placebo), nausea (2.9% on amlodipine, 1.9% on placebo), abdominal pain (1.6% on amlodipine, 0.3% on placebo), and somnolence (1.4% on amlodipine, 0.6% on placebo); these side effects did not appear to be dose-dependent.1
Additionally, some side effects were more common in women than in men. Edema, flushing, palpitations, and somnolence were more often reported by women.1 For example, edema was reported 14.6% of women compare to 5.6% of men, flushing was reported by 4.5% of women compared to1.5% of men, palpitations were reported by 3.3% of women compared to 1.4% of men, and somnolence was reported by 1.6% of women compared to 1.3% of men.
Pooled results from 40 double-blind placebo controlled trials (N=2,988) of amlodipine found overall an adverse event rate of 29.8% of amlodipine recipients compared to 22.1% of placebo recipients (p < 0.001).2 Among amlodipine recipients the most reported adverse effects found to be significant were edema [9.8% on amlodipine, 2.3% on placebo (p < 0.001)], fatigue [4.6% on amlodipine, 2.9% on placebo (p<0.05)], and flushing [2.4% on amlodipine, 0.5% on placebo (p<0.001)]. Compared to placebo, the difference in reports of headache (8.1% on amlodipine, 8.1% on placebo), dizziness (3% on amlodipine, 3.4% on placebo), and nausea (2.8% on amlodipine, 1.9% on placebo) were non-significant (p≥0.10). Rates of withdrawal due to side effects were also non-significant, 1.1% in the amlodipine group and 0.7% in the placebo group (p≥0.10). Similar to the previously mentioned study, data from the pooled results also suggest dose dependent side effects. Incidence of edema and flushing were reported at a higher rate among subjects receiving 10 mg daily (31.3%) than among subjects receiving lower doses (19.5%).
Other studies have also shown increased rates of edema in people taking amlodipine. For example, Nissen et al. compared the effects of amlodipine or enalapril with placebo in a double blind, randomized, controlled trial.3 663 men and women aged 32 to 82 years (mean age 57.3 years) were randomized to the amlodipine group. Participants were given 5 mg of amlodipine for two weeks. Those who tolerated the initial dose were instructed to double their dose for the remainder of the study. Peripheral edema occurred in 32.4% of amlodipine treated patients compared to 9.6% of placebo patients. Cough occurred in 5.1% of amlodipine-treated patients and 5.8% in placebo patients. Hypotension was reported in 3.3% of amlodipine-treated patients and 3.2% in placebo patients.
According to the manufacturer, side effects observed in less than 1.0% of patients but greater than 0.1% were cardiovascular (arrhythmia, bradycardia, chest pain, hypotension, peripheral ischemia, syncope, tachycardia, postural dizziness, postural hypotension, vasculitis), nervous (hypoesthesia, peripheral neuropathy, paresthesia, tremor, vertigo, dry mouth, increased sweating), gastrointestinal (anorexia, constipation, dyspepsia, dysphagia, diarrhea, flatulence, pancreatitis, vomiting, gingival hyperplasia), musculoskeletal (arthralgia, arthrosis, muscle cramps, myalgia), psychiatric (sexual dysfunction in men and women, insomnia, depression, nervousness, depression, abnormal dreams, anxiety, depersonalization), respiratory (dyspnea, epistaxis), dermatological (angioedema, erythema multiforme, pruritis, rash), sensory (abnormal vision, conjunctivitis, diplopia, eye pain, tinnitus), urinary (nocturia, alterations in urinary frequency), metabolic (hyperglycemia, thirst), hematopoietic (leukopenia, purpura, thrombocytopenia), and general (allergic reactions, back pain, hot flashes, malaise, pain, weight gain, weight decrease, rigors).1
Side effects observed in less than 0.1% of patients were cardiac failure, pulse irregularity, extra systoles, skin discoloration, urticaria, skin dryness, alopecia, dermatitis, muscle weakness, twitching, ataxia, hypertonia, migraine, cold and clammy skin, apathy, agitation, amnesia, gastritis, increased appetite, loose stools, coughing, rhinitis, dysuria, polyuria, parasomnia, taste perversion, abnormal visual accommodation, and xerophthalmia.1
References
- FDA. Amlodipine Besylate. Drugs at FDA 2007; http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/019787s042lbl.pdf.
- Osterloh I. The safety of amlodipine. American Heart Journal. 1989/11/01 1989;118(5):1114-1120.
- Nissen SE, Tuzcu E, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: The camelot study: a randomized controlled trial. JAMA. 2004;292(18):2217-2225.
- Packer M, O'Connor CM, Ghali JK, et al. Effect of Amlodipine on Morbidity and Mortality in Severe Chronic Heart Failure. New England Journal of Medicine. 1996;335(15):1107-1114.