A 2013 systematic comparison of all clinically used angiotensin receptor blockers (ARBs) found that most ARBs are rapidly absorbed with maximum bioavailability being reached within half an hour to four hours depending on the specific ARB and dose.1 Elimination half-life ranges from 6 to 24 hours.
|
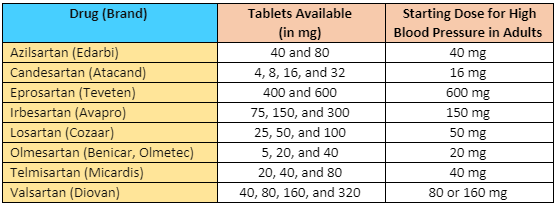
ARB
|
tmax
h
|
t1/2
h
|
Tablets available
|
|
Azilsartan medoxomil
|
1.5 – 3
|
11
|
40 and 80
|
|
Candesartan cilexetil
|
3 – 4
|
9
|
4, 8, 16, and 32
|
|
Eprosartan
|
1 – 2
|
20
|
400 and 600
|
|
Irbesartan
|
1.5 – 2
|
11 – 15
|
75, 150, and 300
|
|
Losartan
|
3 – 4*
|
6 – 9*
|
25, 50, and 100
|
|
Olmesartan medoxomil
|
1 – 2
|
13
|
5, 20, and 40
|
|
Telmisartan
|
0.5 – 1
|
24
|
20, 40, and 80
|
|
Valsartan
|
2 – 4
|
6
|
40, 80, 160, and 320
|
*active metabolite
Table 1: ARB maximum bioavailability and elimination half-life1; Tablets available and dosing from each manufacturer, respectively.2-9
Most of the ARBs control blood pressure for 24 hours with a once daily dosing.10-18 After oral dosing, ARBs are absorbed rapidly (time for peak plasma levels = 0.5 – 4 hours) with a wide range of bioavailability (13% for eprosartan to 60 – 80% for irbesartan).19
Multiple sources indicate that angiotensin receptor blockers (ARBs) may be administered with or without food.8,20
Valsartan is the only ARB that is affected by food. Food may decrease the rate and extent of valsartan absorption by about 40%.1,2,19,21 However, the manufacturers state that the drug can be taken without regard to food.2
References
- Michel MC, Foster C, Brunner HR, Liu L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol Rev 2013; 65 (2): 809-848.
- Diovan [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2017.
- Edarbi [package insert]. Atlanta, GA: Arbor Pharmaceuticals, LLC; 2016.
- Atacand [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
- Avapro [package insert]. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2016.
- Cozaar [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; 2015.
- Teveten [package insert]. North Chicago, IL: AbbVie Inc.; 2014.
- Micardis [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2014.
- Benicar [package insert]. Parsippany, NJ: Daiichi Sankyo, Inc; 2009.
- Carr AA, Prisant LM. Losartan: first of a new class of angiotensin antagonists for the management of hypertension. J Clin Pharmacol 1996; 36 (1): 3-12.
- Ogihara T, Arakawa K. Clinical efficacy and tolerability of candesartan cilexetil. Candesartan Study Groups in Japan. J Hum Hypertens 1999; 13 Suppl 1: S27-31; discussion S33-24.
- Gillis JC, Markham A. Irbesartan. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in the management of hypertension. Drugs 1997; 54 (6): 885-902.
- Markham A, Goa KL. Valsartan. A review of its pharmacology and therapeutic use in essential hypertension. Drugs 1997; 54 (2): 299-311.
- McClellan KJ, Balfour JA. Eprosartan. Drugs 1998; 55 (5): 713-718; discussion 719-720.
- McClellan KJ, Markham A. Telmisartan. Drugs 1998; 56 (6): 1039-1044; discussion 1045-1036.
- Hedner T, Oparil S, Rasmussen K, et al. A comparison of the angiotensin II antagonists valsartan and losartan in the treatment of essential hypertension. Am J Hypertens 1999; 12 (4 pt 1): 414-417.
- Oparil S, Guthrie R, Lewin AJ, et al. An elective-titration study of the comparative effectiveness of two angiotensin II-receptor blockers, irbesartan and losartan. Irbesartan/Losartan Study Investigators. Clin Ther 1998; 20 (3): 398-409.
- Smith DH. Comparison of angiotensin II type 1 receptor antagonists in the treatment of essential hypertension. Drugs 2008; 68 (9): 1207-1225.
- Israili ZH. Clinical pharmacokinetics of angiotensin II (AT1) receptor blockers in hypertension. J Hum Hypertens 2000; 14 Suppl 1: S73-86.
- Cozaar [package insert]. Kirkland, QC Canada: Merck Canada, Inc; 2014.
- Chiolero A, Burnier M. Pharmacology of valsartan, an angiotensin II receptor antagonist. Expert Opin Investig Drugs 1998; 7 (11): 1915-1925.

.png)