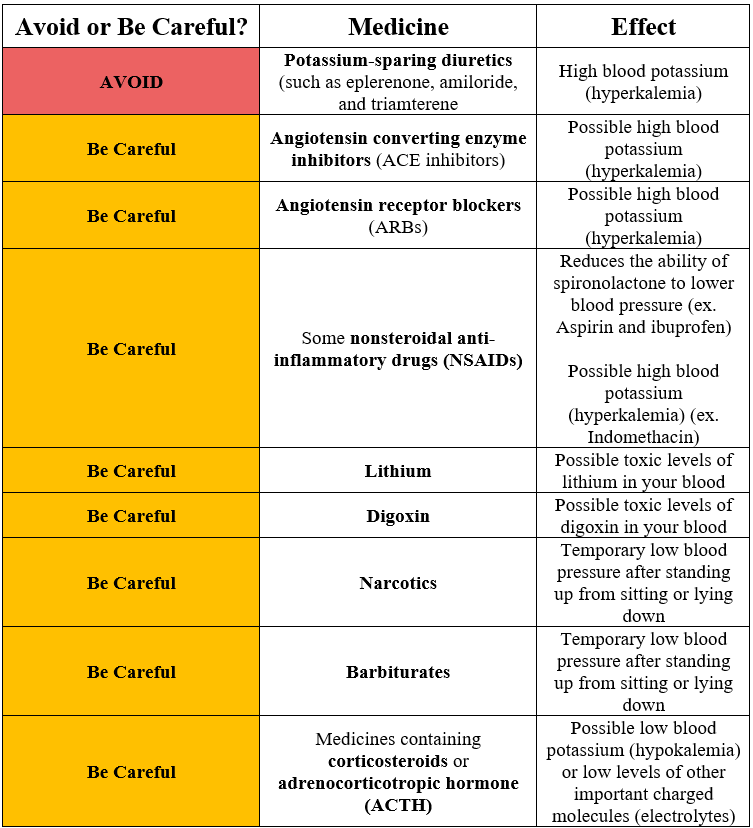
Due to the potassium-sparing nature of spironolactone, co-administration with some prescription medications may lead to hyperkalemia (serum potassium >5.0 mmol/L).1-9
According to the Food and Drug Administration (FDA), use of spironolactone with other potassium-sparing diuretics, such as eplerenone, amiloride, and triamterene is contraindicated.2-4
The FDA also cautions against the co-administration of spironolactone with either angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs).2 However, the American College of Cardiology and American Heart Association (ACC/AHA) guidelines indicate that the combination (either ACE inhibitor or ARB with spironolactone) is appropriate treatment for heart failure patients with reduced or preserved ejection fraction.7 The ACC/AHA also recommends against the triple combination of spironolactone, ACE inhibitor, and ARB.7
The placebo-controlled Randomized Aldactone Evaluation Study (RALES) examined the addition of spironolactone in 214 heart failure patients already being treated with an ACE inhibitor and loop diuretic.1 Patients in the treatment group were randomized to receive 12.5, 25, 50, or 75 mg of spironolactone per day. The incidence of hyperkalemia appeared to be dose-dependent as 5% of patients in the 12.5 mg group developed hyperkalemia compared to 24% of patients in the 75 mg group. In the placebo group, 5% of patients developed hyperkalemia. Researchers concluded that the combination of spironolactone (>50 mg) and high doses of an ACE inhibitor (captopril ≥75 mg, enalapril ≥10 mg, or lisinopril ≥10 mg) increased the risk of developing hyperkalemia.
In addition to randomized controlled trials, real-world studies have also reported hyperkalemia-related hospitalizations and mortality with the co-administration of spironolactone and an ACE inhibitor.5,6 Researchers studied the increase of new spironolactone prescriptions to elderly patients already using an ACE inhibitor for severe heart failure in Ontario, Canada after publication of the RALES trial.6 From 1999 to 2001, spironolactone prescription rates (per 1,000) increased fivefold (30 to 149, respectively, p<0.001). During this period, there was a threefold increase in the rate of hyperkalemia-related hospitalization rates (4.0 to 11.0, p<0.001) and deaths (0.7 to 2.0, p<0.001).
The risk of hyperkalemia from the co-administration of spironolactone and candesartan, an ARB, was evaluated in a follow up study of patients in the Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity (CHARM) Program.9 For patients on spironolactone, the odds were higher for clinically important hyperkalemia events (dose reduction, study drug discontinuation, hospitalization, or death) in those who were randomized to receive candesartan (59 of 643, 9.2%) compared to placebo (12 of 643, 1.9%) (p=0.04).
The concomitant use of nonsteroidal anti-inflammatory drugs (NSAIDs) and spironolactone has been associated with severe hyperkalemia.2 In particular, indomethacin has been shown to increase serum potassium even when administered alone.8 In a case analysis of 50 admitted patients who were administered indomethacin (mean dose 172 mg/d), there was an observed increase in the serum potassium in all patients.8 Indomethacin induced hyperkalemia in 23 of these patients.
Besides the risk of drug-induced hyperkalemia, there are other unintended effects with the combination of spironolactone and some prescription medications.2 For example, NSAIDs may also reduce the natriuretic, diuretic, and antihypertensive effect of loop, potassium-sparing, and thiazide diuretics in some patients.2 In a meta-analysis of 38 randomized, placebo-controlled trials, NSAIDs raised supine mean arterial pressure (MAP) by 5.0 mm Hg (p<0.05).10 In particular, piroxicam, indomethacin and ibuprofen induced the most marked increases in supine MAP. Aspirin, sulindac, and flurbiprofen induced smaller increases supine MAP. Patients treated with antihypertensives experienced a greater increase in blood pressure than those who were not taking antihypertensives (4.7 mm Hg compared to 1.8 mm Hg, p<0.05).
Spironolactone also reduces the renal clearance of lithium and digoxin, leading to an increased risk of lithium or digoxin toxicity.11-15 As a result, the FDA recommends against concurrent use of spironolactone and lithium.2 For digoxin, the FDA recommends careful monitoring of the patient and reducing the dose of digoxin when co-administered with spironolactone if necessary.
Current FDA recommendations caution against the use of spironolactone with narcotics or barbiturates to avoid the potentiation of orthostatic hypertension (OH).2 These medications have been shown to induce OH when administered alone.2,16,17 For example, researchers examined hypertensive and normotensive participants to determine the prevalence of OH.17 In hypertensive participants, those taking spironolactone were more likely to experience OH than those who were not taking spironolactone (odds ratio [OR]=3.29, p=0.003).
According to the FDA, a combination of spironolactone and medications containing corticosteroids or adrenocorticotropic hormone (ACTH) may result in intensified electrolyte depletion and in particular, hypokalemia.2 However, some studies have actually shown an increase serum potassium with this combination.18,19
References
- Pitt B, et al; The Rales Investigators. Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (The Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol. 1996;78(8):902-907.
- Aldactone [package insert]. New York, NY: Pfizer, Inc.; 2018.
- Dyrenium [package insert]. Sarasota, FL: WellSpring Pharmaceutical Corporation; 2009.
- Amiloride Hydrochloride [package insert]. Bensalem, PA: Sigmapharm Laboratories LLC; 2009.
- Bozkurt B, Agoston I, Knowlton AA. Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines. J Am Coll Cardiol. 2003;41(2):211-214.
- Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543-551.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128(16):e240-e327.
- Zimran A, Kramer M, Plaskin M, Hershko C. Incidence of hyperkalaemia induced by indomethacin in a hospital population. Br Med J. 1985;291(6488):107-108.
- Desai AS, Swedberg K, McMurray JJV, et al. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM program. J Am Coll Cardiol. 2007;50(20):1959-1966.
- Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med. 1994;121(4):289-300.
- Baer L, Platman SR, Kassir S, Fieve RR. Mechanisms of renal lithium handling and their relationship to mineralocoticoids: A dissociation between sodium and lithium ions. J Psychisatr Res. 1971 1971;8(2):91-105.
- Finley PR. Drug interactions with lithium: an update. Clinl Pharmacokin. 2016;55(8):925-941.
- Lanoxin [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2012.
- Steiness E. Renal tubular secretion of digoxin. Circulation. 1974;50(1):103-107.
- Thomsen K, Schou M. Renal lithium excretion in man. Am J Physiol. Oct 1968;215(4):823-827.
- Grubb BP. Neurocardiogenic syncope and related disorders of orthostatic intolerance. Circulation. 2005;111(22):2997-3006.
- Fedorowski A, Burri P, Melander O. Orthostatic hypotension in genetically related hypertensive and normotensive individuals. J Hypertens. 2009;27(5):976-982.
- Reis Gv, Liljestrand Å, Matell G. Results with acth and spironolactone in severe cases of myasthenia gravis. Acta Neurol Scand. 1965;41(S13):463-472.
- Gaillard RC, Riondel AM, Favrod-Coune CA, Vallotton MB, Muller AF. Aldosterone escape to chronic ACTH administration in man. Acta Endocrinol. 1983;103(1):116-124.