Abatacept is administered intravenously or subcutaneously as a treatment for rheumatoid arthritis (RA) either alone or in combination with other disease-modifying antirheumatic drugs (DMARDs).
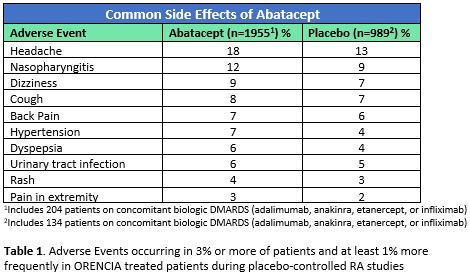
In clinical trials, abatacept by either route was generally well tolerated compared to placebo with the most common side effects being headache, nasopharyngitis, dizziness, cough, back pain, hypertension, dyspepsia, urinary tract infection, rash, and pain in the extremities.1,2 The frequency of each is described in Table 1.
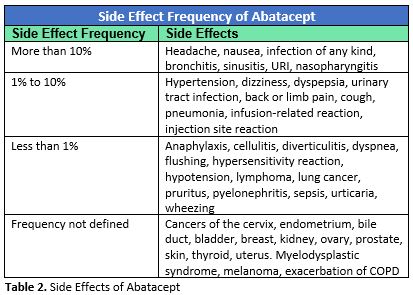
Drug Information compiled in clinical trials (including open-label, non-placebo-controlled trials) before abatacept’s release reported the following side effects and their frequency:3
In a double-blind study that compared 232 patients taking abatacept plus methotrexate (MTX) with 227 taking MTX alone, Westhovens et al. reported the side effect profile was similar in both groups (84.8% vs 83.4%), suggesting that the addition of abatacept did not contribute significantly to the side effect profile.4
Infections
According to the manufacturer’s package insert, infections occurred more frequently in patients treated with abatacept (54%) compared to placebo-treated patients (48%) in placebo-controlled clinical trials.2 The most common infections (reported in 5% to 13% of abatacept users) were upper respiratory infection (URI), nasopharyngitis, sinusitis, urinary tract infections, influenza, and bronchitis. Infections that were reported in fewer than 5% of abatacept users were rhinitis, herpes simplex, and pneumonia. Infections were the most frequently cited reason for drug discontinuation or interruption.2
In a pooled analysis of eight clinical trials (total n= 4,149), the incidence rate of serious infections per 100 patient-years (PY) of exposure to intravenous abatacept was 2.87 (95% confidence interval [CI] 2.57 – 3.19).5 The most common infection in this study was pneumonia.
Patients with RA have a higher rate of infections than patients without RA. To better understand the infection rate in patients taking abatacept, Simon et al. reviewed rates of serious infection from seven abatacept clinical trials (n=6,089) and compared them to serious infection rates reported by RA patients taking other non-biologic DMARDS from six reference RA cohorts (n>133,000).6 The rate of infections that required hospitalization was higher among patients receiving abatacept (3.05 per 100 PY) compared to exposure to other DMARDS (2.15 per 100 PY), but the rate ratio was not statistically significant (rate ratio 1.42; 95% CI [0.82 – 2.45]).
In the Orencia and Rheumatoid Arthritis registry (ORA), a five-year prospective registry of RA patients in France, the serious infection rate was slightly higher in the 976 patients treated with abatacept than had been reported in clinical trials (4.1 per 100 PY exposure).7 A serious infection was defined as one requiring hospitalization, intravenous antibiotics, or resulting in death. A previous history of serious infection was significantly associated with a higher risk of serious infection while taking abatacept (hazard ratio 1.94, 95% CI [1.18 – 3.20], p=0.009).
Malignancy
In placebo-controlled clinical trials (n=1,955 treated for a median of 12 months), overall malignancy rates were similar between those people treated with abatacept (1.3%) and controls (1.1%).2 Four individuals taking abatacept in these trials developed lung cancer (0.2%) compared to none of those taking a placebo. In the cumulative clinical trials that included uncontrolled and open-label trials (n=2,688, 3,827 PY) eight instances (0.21 per 100 PY) of lung cancer and four lymphomas (0.10 per 100 PY) occurred in people taking abatacept. The lymphoma rate is 3.5 times the national rate for individuals of the same age and gender.2 However, people with RA are at higher risk of lung cancer and lymphoma compared to the general population.8 Simon et al. reported the standard incidence rate of lung cancer in patients with RA compared with the general population to be 1.63 (95% CI [1.43 – 1.87]), and for lymphoma to be 3.21 (95% CI [2.42 – 4.27]). The role of abatacept in the development of malignancy is not known.9
Infusion-Related and Hypersensitivity Reactions
Infusion-related events, defined as adverse events that occur within an hour of starting an intravenous medication, were more common in people taking abatacept (9%) than placebo (6%) in three randomized placebo-controlled clinical trials.2 Between 1% and 2% of those given abatacept experienced dizziness, headache, or hypertension. Fewer than 1% experienced hypotension, dyspnea, nausea, flushing, urticaria, cough, hypersensitivity, pruritus, rash, or wheezing. Fewer than 1% discontinued abatacept because of an infusion-related event. Anaphylaxis following infusion with abatacept was rare (<0.1%). Other drug reactions that occurred within 24 hours of infusion including hypotension, urticaria, and dyspnea were uncommon (<1%).
Weinblatt et al. pooled data from six abatacept clinical trials (n=3,755, 9,662 PY of exposure). The incidence rate of infusion-related events per 100 PY exposure to abatacept was 3.9.5 The events became less common with time, usually occurring within the first few months of treatment.
Abatacept and COPD
In clinical trials, people with chronic obstructive pulmonary disease (COPD) who received abatacept had a higher frequency of adverse reactions (97%) including exacerbation of their COPD, pneumonia, cough, rhonchi, and shortness of breath, than people with COPD taking placebo (88%).2,3 Suissa et al., however, conducted a matched cohort trial and found no increased risk of respiratory events among patients with RA and COPD taking abatacept (n=1807) compared to those not taking abatacept (3547).10 Although the package insert recommends caution using abatacept in people with COPD and close monitoring of respiratory status, current data does not support an absolute or relative contraindication of abatacept among patients with RA and COPD.
References
- Genovese MC, Covarrubias A, Leon G, et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum 2011; 63 (10): 2854-2864.
- Orencia (Abatacept) package insert. Princeton, NJ: Bristol-Myers Squib; 2013.
- Abatacept: Drug Information. Lexicomp, Inc, Wolters Kluwer Health, Inc, Riverwoods, IL. http//:online.lexi.com. Accessed November 2, 2019.
- Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis 2009; 68 (12): 1870-1877.
- Weinblatt ME, Moreland LW, Westhovens R, et al. Safety of abatacept administered intravenously in treatment of rheumatoid arthritis: integrated analyses of up to 8 years of treatment from the abatacept clinical trial program. J Rheumatol 2013; 40 (6): 787-797.
- Simon TA, Askling J, Lacaille D, et al. Infections requiring hospitalization in the abatacept clinical development program: an epidemiological assessment. Arthritis Res Ther 2010; 12 (2): R67.
- Salmon JH, Gottenberg JE, Ravaud P, et al. Predictive risk factors of serious infections in patients with rheumatoid arthritis treated with abatacept in common practice: results from the Orencia and Rheumatoid Arthritis (ORA) registry. Ann Rheum Dis 2016; 75 (6): 1108-1113.
- Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther 2015; 17 : 212.
- Blair HA, Deeks ED. Abatacept: a review in rheumatoid arthritis. Drugs 2017; 77 (11): 1221-1233.
- Suissa S, Hudson M, Dell'Aniello S, et al. Comparative safety of abatacept in rheumatoid arthritis with COPD: a real-world population-based observational study. Semin Arthitis Rheum 2019; 49(3): 366-372.



.png)

