Side Effect Monitoring and Dose Titration
When metformin is newly initiated, patients need regular close follow-up with their healthcare providers for dose titration and assessment of side effects, medication adherence, and barriers to effective self-management.1 Metformin is initiated at a low dose and titrated every one to two weeks based on tolerability.2 The frequency of these initial visits will depend on patient and physician factors. Once a stable dose has been reached, patients need follow-up visits at least every three months until glycemic goals are reached and at least every 6 to 12 months thereafter.1 These follow-up visits should include evaluation for new complications or comorbidities, discussion of behavioral and lifestyle factors (including eating patterns, weight, and physical activity), assessment of medication intolerance or side effects, and discussion of medication-taking behavior. Decisions about ongoing management should be made using shared decision-making between patient and provider.
Blood Glucose Monitoring
According to the 2021 “Standards of Medical Care in Diabetes” from the American Diabetes Association (ADA), patients should have their hemoglobin A1C (A1C) checked quarterly after any change in their diabetes therapy, including after starting metformin, or as their metformin dose is being titrated.3 According to manufacturers’ data, A1C should be checked every three months until blood glucose goals are met.1 Once glucose goals are met, the A1C should be repeated at least twice per year.3 Hemoglobin A1C is a test used to measure average glycemia. Specifically, the A1C test measures the percent of hemoglobin to which glucose is bound, and this measurement reflects the average blood glucose over the past three months.4 The A1C goal for most adults is less than 7%. However, the goal can vary among patients based on age and disease factors.1
Additional methods to assess glycemic control, such as patient self-monitoring of blood glucose (SMBG) or continuous glucose monitoring (CGM) may be useful for some patients, but are not typically used in patients on metformin alone.3 These methods are primarily used for patients who are taking insulin.
Renal Function Monitoring
Metformin is excreted by the kidneys. Severe renal insufficiency, either due to stage 4 chronic kidney disease or acute kidney injury (eGFR <30 mL/minute/1.73 m2) is a contraindication to the use of metformin.5,7 Accumulation of metformin due to renal insufficiency increases the risk of lactic acidosis, a rare but life-threatening side effect.6,7 Renal function must be assessed initially when a patient is started on metformin and periodically thereafter, with the frequency of monitoring based on previous renal function and risk factors, as follows:
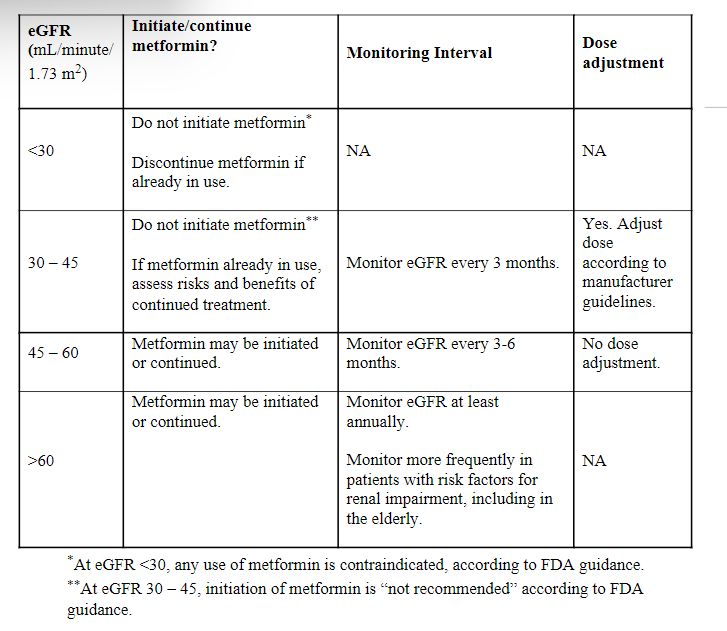
Renal function monitoring is essential, as metformin accumulation in patients with renal failure can cause lactic acidosis, a very rare metabolic complication. According to manufacturers’ data, the incidence of lactic acidosis is estimated at 0.03 cases/1000 patient-years.5 Lactic acidosis is characterized by elevated blood lactate levels (>5 mmol/L), leading to a decrease in blood pH with an associated anion gap, and an increase in the lactate/pyruvate ratio. Lactic acidosis can lead to hypothermia, hypotension, bradyarrhythmia, and death.
Monitoring of Vitamin B12 and Hematologic Parameters
Monitoring of vitamin B12 and other hematologic parameters is recommended in patients taking metformin, because metformin use has been associated with B12 deficiency and resulting anemia.5 Some drug manufacturers suggest checking vitamin B12 levels and hematological parameters such as hemoglobin, hematocrit, and red blood cell size annually.2 Others suggest checking every two to three years.5
Vitamin B12 levels were assessed on the Diabetes Prevention Program Outcomes Study cohort, one of the largest and longest studies of metformin treatment.8 This randomized controlled trial enrolled patients who were at high risk for diabetes and assigned them to metformin versus intensive lifestyle intervention versus placebo. The study found that after five years of taking metformin twice a day, the average B12 levels were 10% lower in the metformin group (n=858) as compared to the placebo group (n=857). The prevalence of vitamin B12 deficiency in the metformin group was 4.3%, compared to 2.3% in the placebo group (p=0.02).
In a 2018 randomized controlled trial, participants (n=390) with type 2 diabetes received either metformin or a placebo for 4.3 years.9 The study evaluated methylmalonic acid (MMA), a biomarker which is increased in B12 deficiency. Metformin was found to increase MMA by 0.039 μmol/L (95% confidence interval [CI] [0.019 – 0.055], p=0.001), compared to baseline levels of >220pmol/L.
Monitoring of Hepatic Function
Hepatic function is also relevant to the use of metformin, as liver disease has been associated with an increased risk of lactic acidosis.1 According to the ADA, metformin “should be used with caution in patients with impaired hepatic function.” 3 Current guidelines do not specify a recommended frequency for monitoring of hepatic function.
Comprehensive Diabetes Medical Evaluation
To evaluate for diabetes complications and comorbid conditions, there are additional tests that are needed regularly for all patients with diabetes, including those taking metformin.
Diabetes increases cardiovascular risk10. Close monitoring of cardiovascular risk factors such as hypertension and dyslipidemia is recommended. The ADA recommends that blood pressure be checked regularly, as hypertension is a risk factor for atherosclerotic cardiovascular disease and microvascular complications. The ADA also recommends checking a lipids profile (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides) at the time of initial diagnosis, and subsequently every one to five years depending on age and lipid levels.
One of the microvascular complications that diabetes can cause is kidney disease.11 Urinary microalbuminuria is the earliest clinical finding of kidney disease. A urine test for microalbuminuria is recommended at least annually in patients with diabetes for early detection of kidney disease.
Foot ulcers are common in patients with diabetes as a result of diabetic neuropathy and peripheral arterial disease.12 As a result, all adults with diabetes should undergo a foot examination annually to assess skin integrity, musculoskeletal deformities, and sensation.
Diabetic retinopathy is another vascular complication that occurs in patients with diabetes.12 Diabetic retinopathy can cause blindness, glaucoma, cataracts, and other disorders of the eye. Patients with type 1 diabetes should have an eye exam within five years of diabetes onset, and patients with type 2 diabetes should have an eye exam at the time of diagnosis. If there is no evidence of retinopathy, screening is recommended every one to two years. If there is evidence of retinopathy, exams should be repeated at least annually.
Periodontal disease is associated with poor glycemic control, so it is important for patients with diabetes to have oral exams at least annually.13
References
- MetFORMIN Monograph for Professionals. Drugs.com. https://www.drugs.com/monograph/metformin.html
- MetFORMIN Drug Monographs. Monitoring. Pennsylvania Plaza New York City: McGraw Hill Medical; 2021.
- 6. Glycemic Targets:Standards of Medical Care in Diabetes—2021. Diabetes Care. 2017;41(Supplement 1): S55-S64. doi:10.2337/dc18-s006
- Nicoll D, Chuanyi Mark Lu, Mcphee SJ. Guide to Diagnostic Tests. Mcgraw-Hill Education; 2017.
- Enforcement Reports. www.accessdata.fda.gov. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020357s037s039
- Lipska KJ, Bailey CJ, Inzucchi SE. Use of Metformin in the Setting of Mild-to-Moderate Renal Insufficiency. Diabetes Care. 2011;34(6):1431-1437. doi:10.2337/dc10-2361
- Research C for DE and. FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. FDA. Published online February 9, 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-warnings-regarding-use-diabetes-medicine-metformin-certain
- Aroda VR, Edelstein SL, Goldberg RB, et al. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. The Journal of Clinical Endocrinology & Metabolism. 2016;101(4):1754-1761. doi:10.1210/jc.2015-3754
- Out M, Kooy A, Lehert P, Schalkwijk CA, Stehouwer CDA. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: Post hoc analysis of a randomized controlled 4.3 year trial. Journal of Diabetes and its Complications. 2018;32(2):171-178. doi:10.1016/j.jdiacomp.2017.11.001
- American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2020;44(Supplement 1):S125-S150. doi:10.2337/dc21-s010
- FarkouhE., & Rayfield E.J., & Fuster V (2017). Diabetes and cardiovascular disease. Fuster V, & Harrington R.A., & Narula J, & Eapen Z.J.(Eds.), Hurst's The Heart, 14e. McGraw Hill. https://accessmedicinemhmedical.com.ezproxy.med.cornell.edu/content.aspx?bookid=2046§ionid=176573935
- Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2020;44(Supplement 1):S151-S167. doi:10.2337/dc21-s011
- Working Together to Manage Diabetes: A Toolkit for Pharmacy, Podiatry, Optometry, and Dentistry. Accessed September 19, 2022. https://www.cdc.gov/diabetes/ndep/pdfs/working_together_to_manage_diabetes_webinar_slides.pdf


